In Vitro Cell Behavior and Antibiotic Activity under Sustained Release of Doxycycline-Loaded Poly(lactic-co-glycolic acid) Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PLGA and PLGA + DOX Microspheres
2.2. Microspheres Characterization
2.2.1. Fourier Transform Infrared (FT−IR)
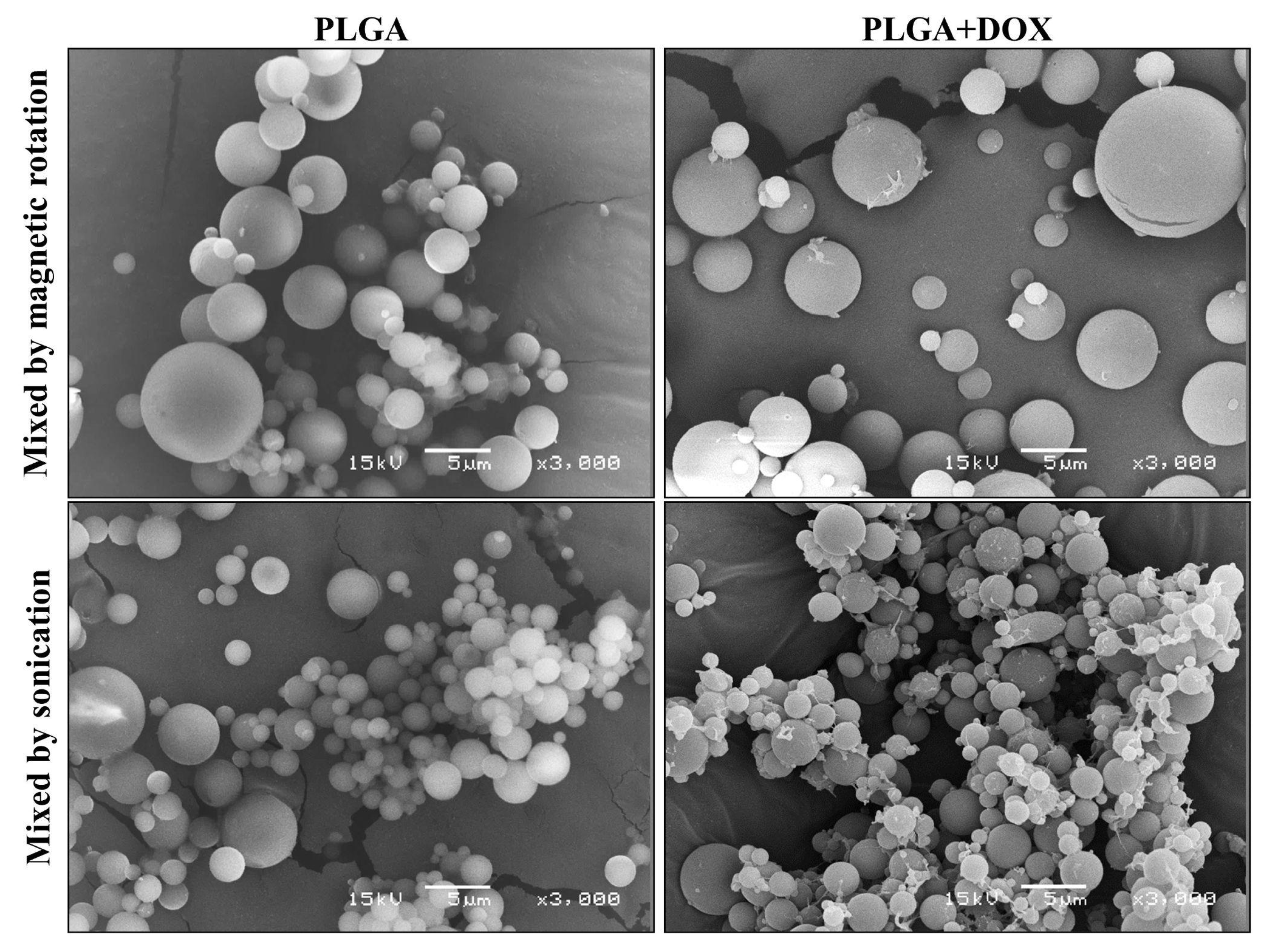
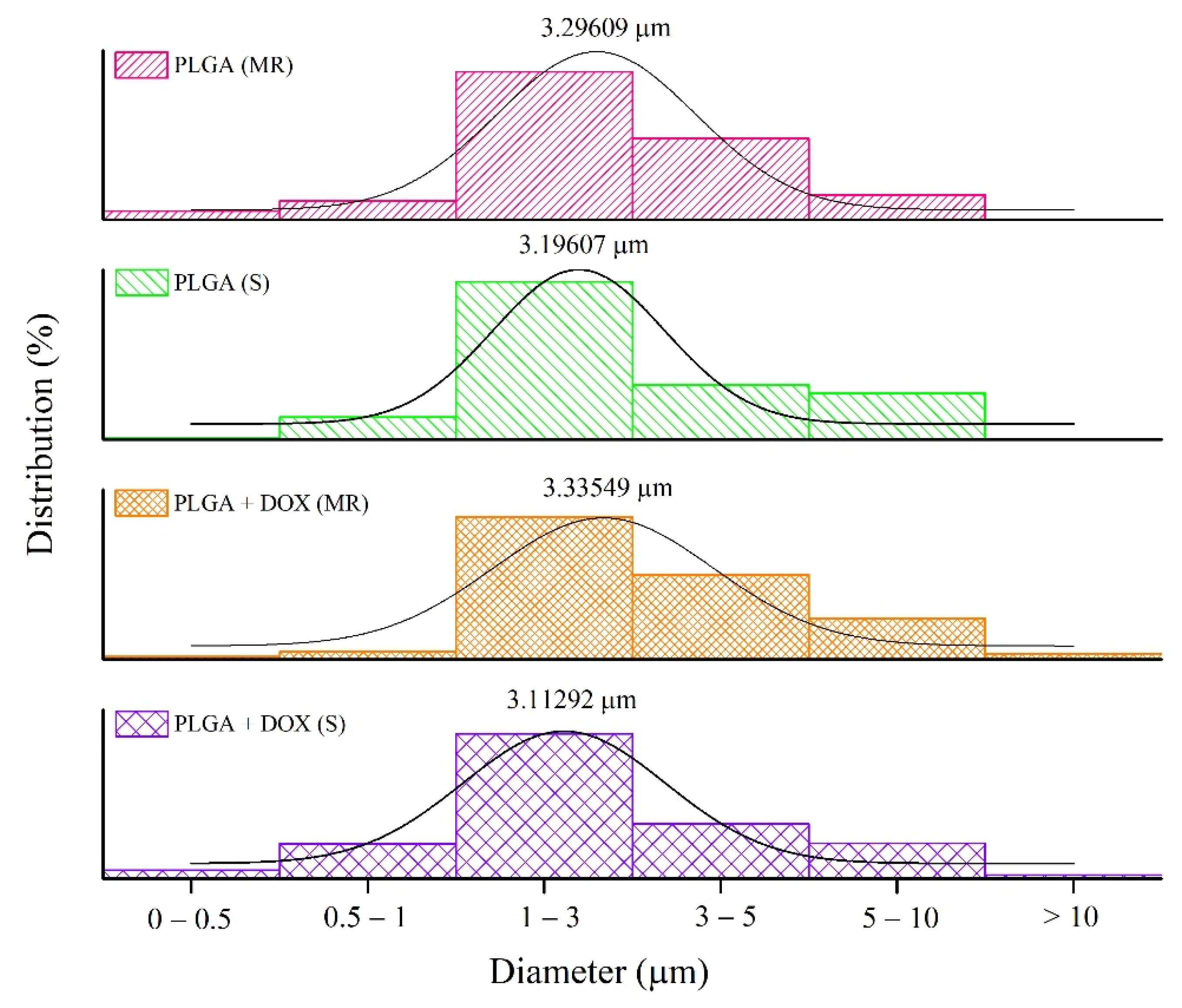
2.2.2. Morphology and Particle Size Distribution
2.2.3. Encapsulation Efficiency (EE) and Drug Loading (DL)
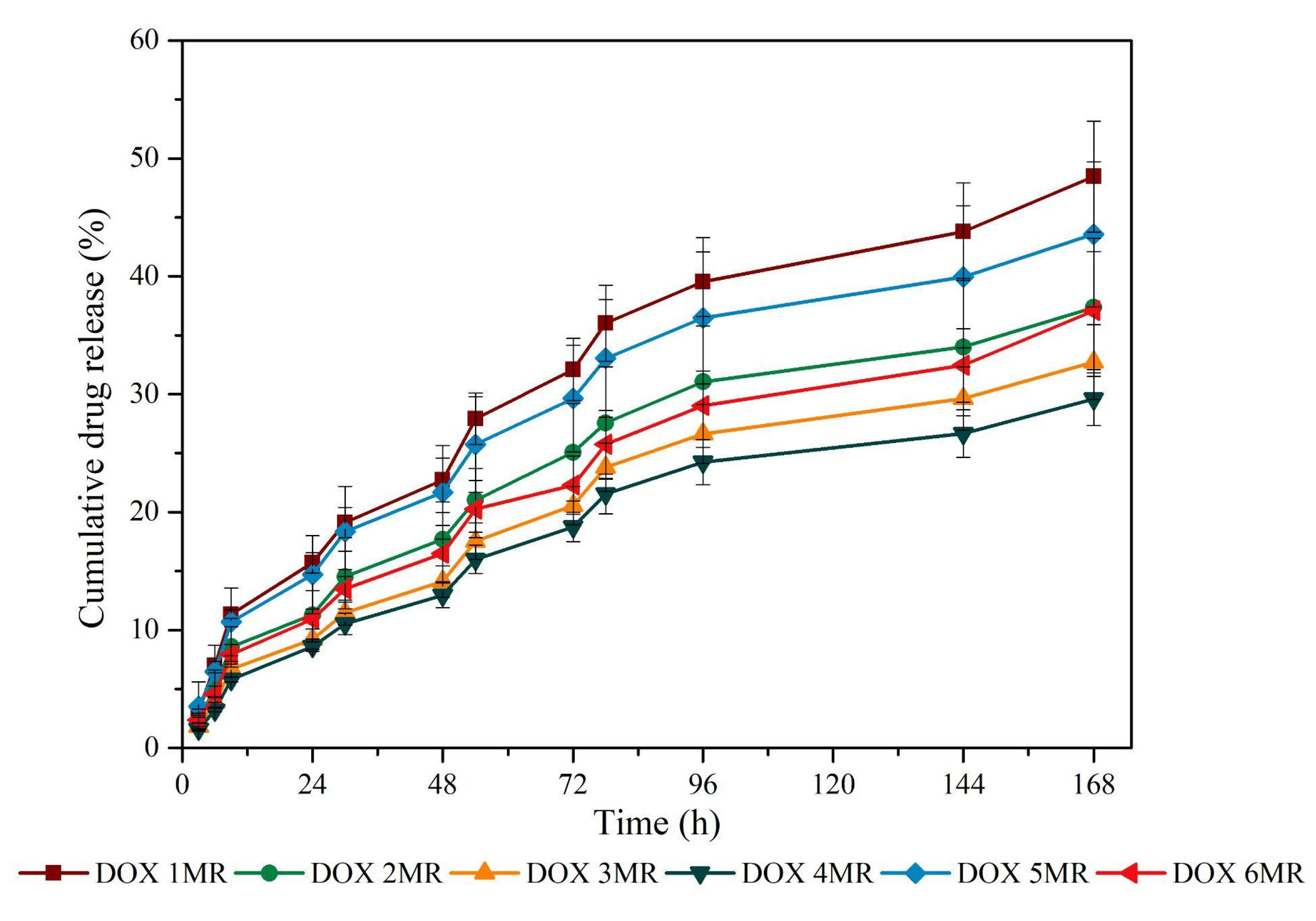
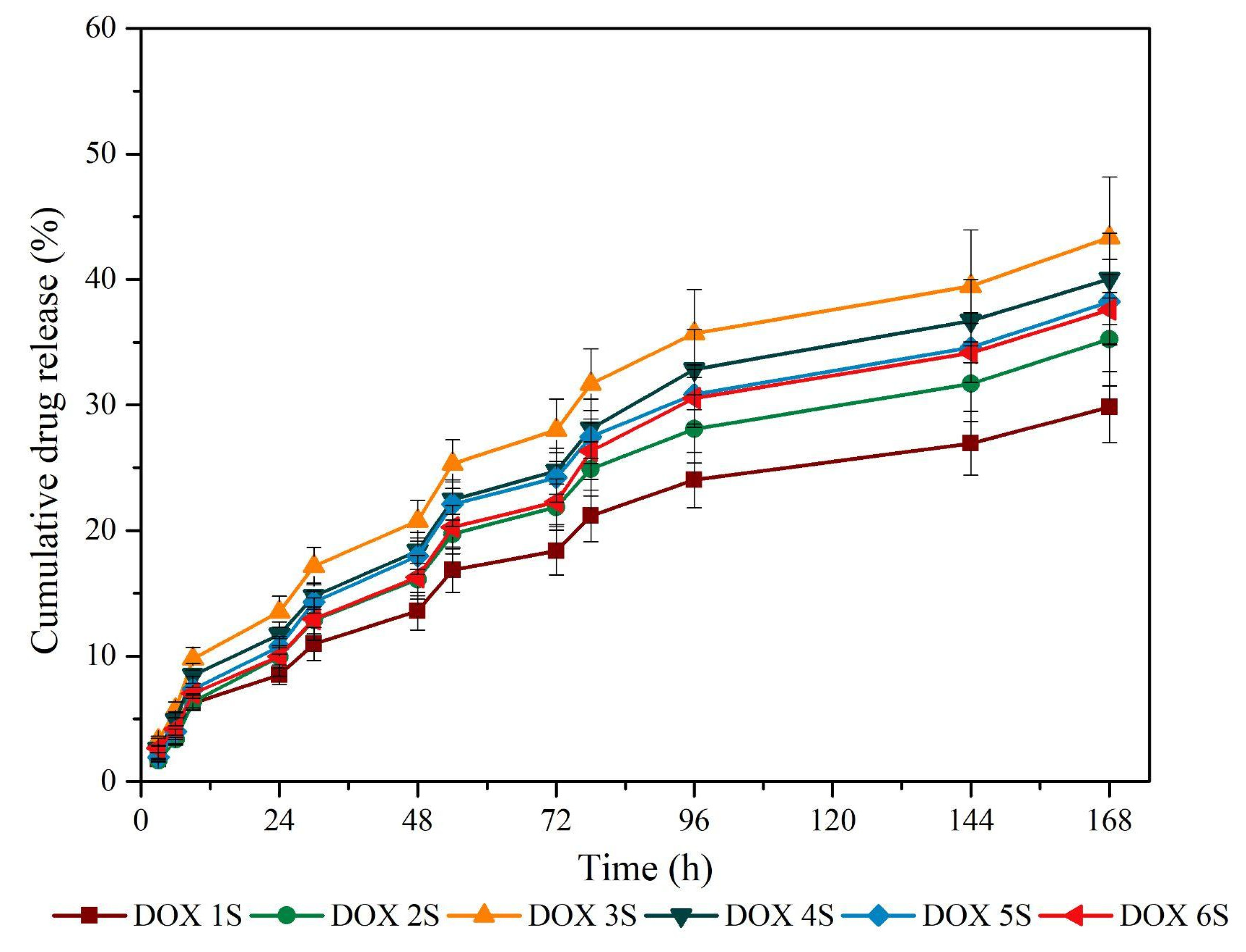
2.2.4. Doxycycline Release Test
2.3. In Vitro Antimicrobial Activity
2.4. In Vitro Biocompatibility
2.4.1. Viability by MTT Assay
2.4.2. Cell Survival and Morphology
2.5. Statistical Analysis
3. Results
3.1. FT−IR
3.2. Microsphere Measurements
3.3. Encapsulation Efficiency
3.4. Doxycycline Release Profile
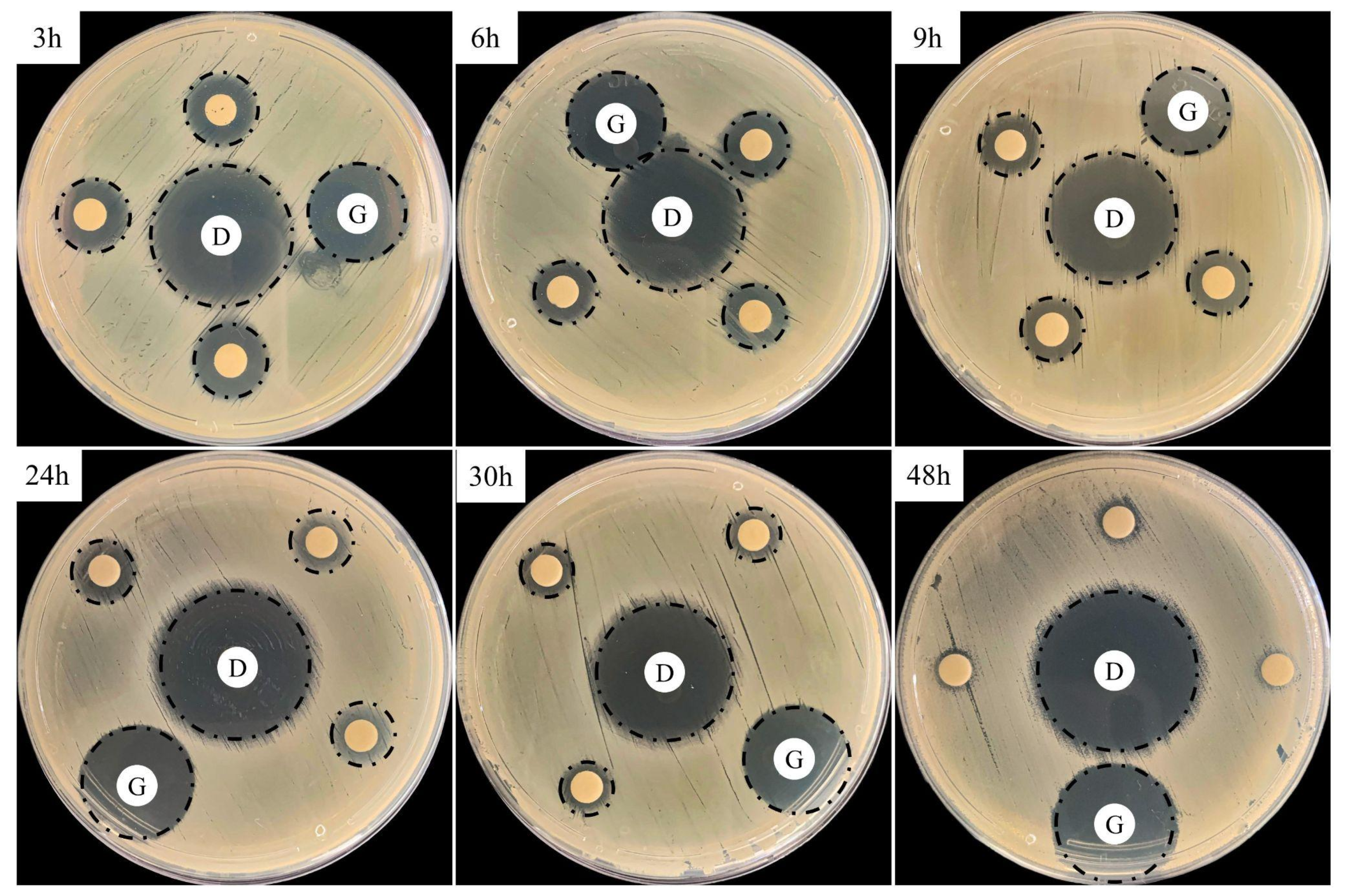
3.5. In Vitro Antimicrobial Activity
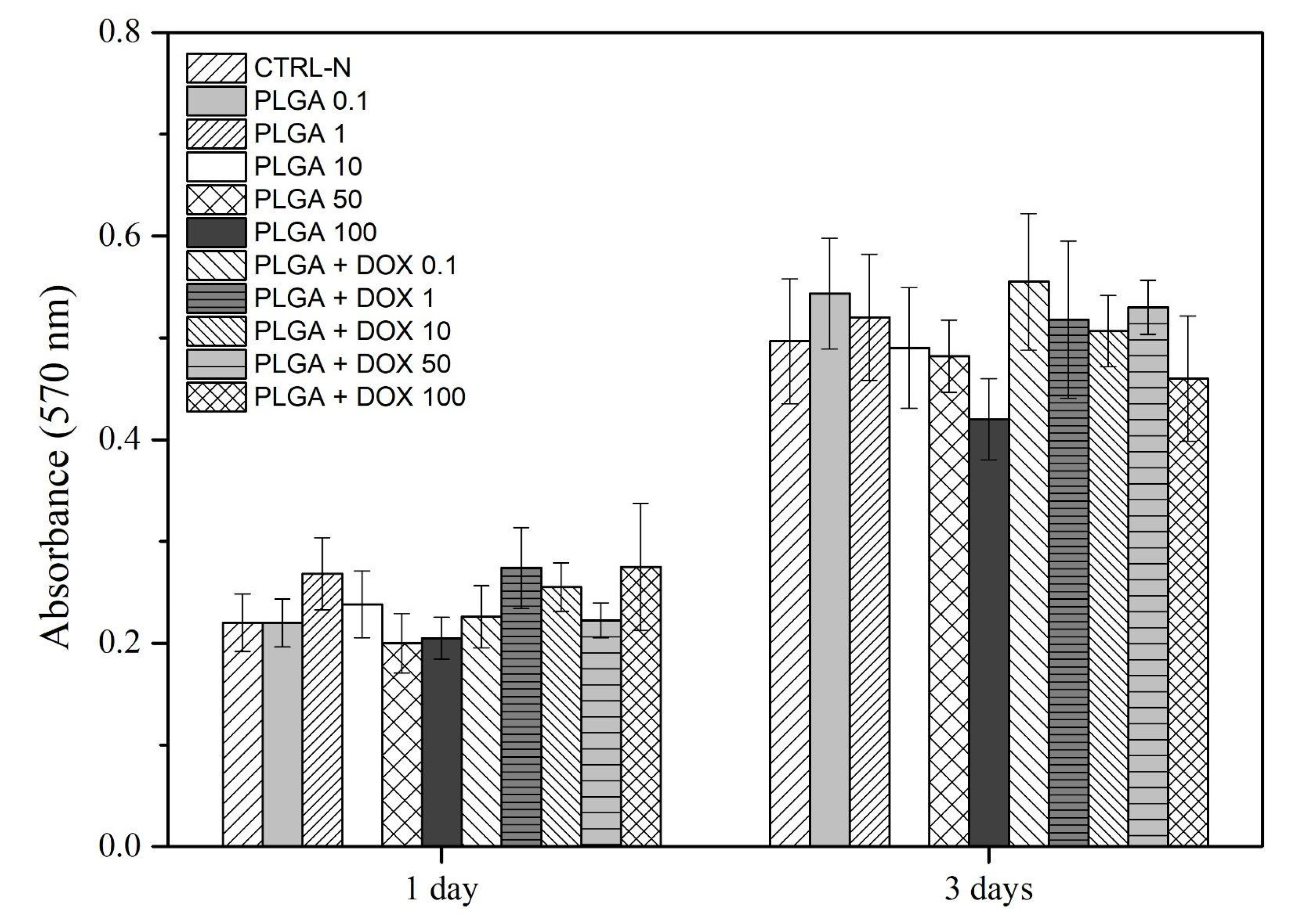
3.6. Cell Viability by Metabolic Activity
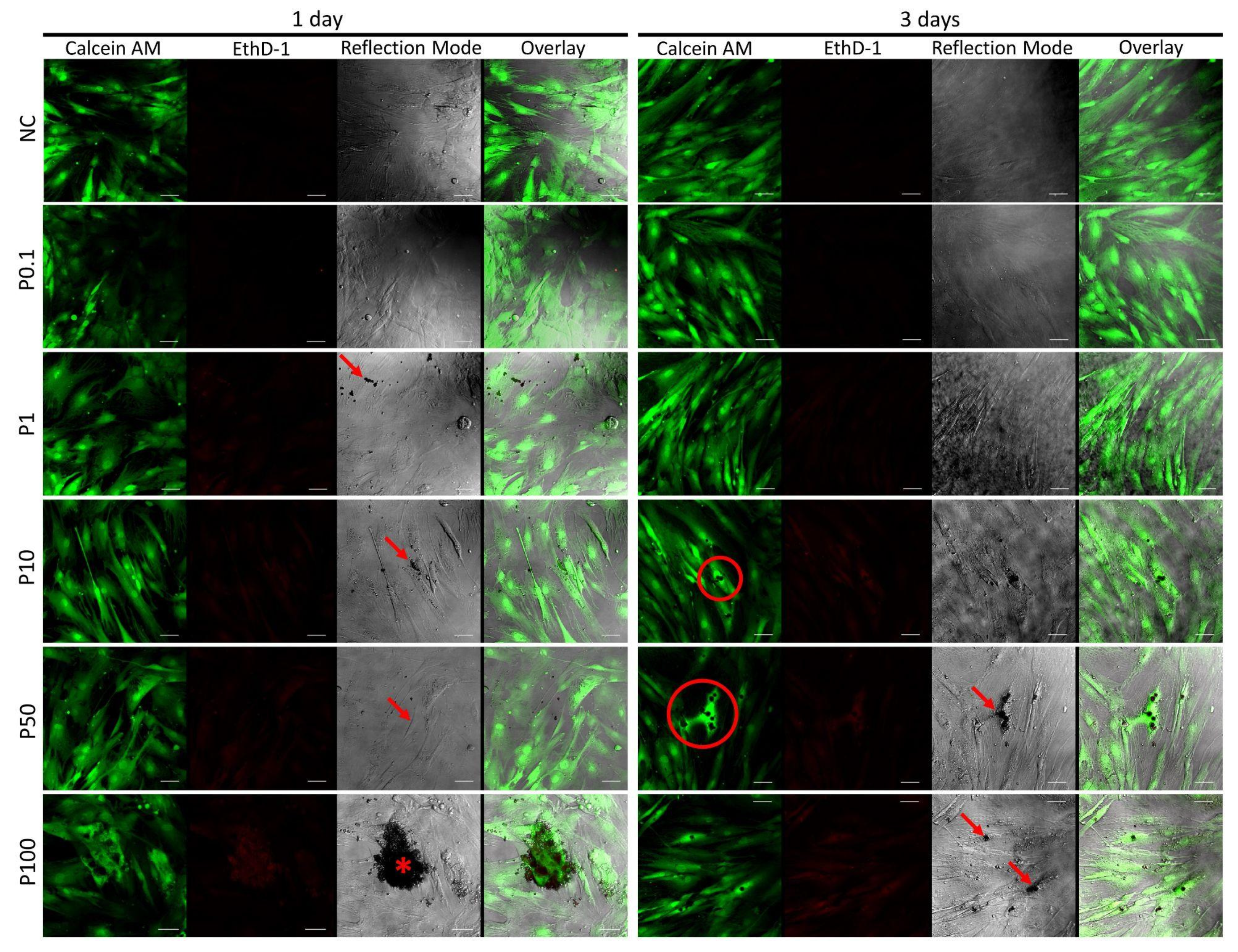
3.7. Cell Survival and Morphology by Live/Dead Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jain, K.K. An overview of drug delivery systems. In Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2059, pp. 1–54. [Google Scholar]
- Liu, H.; Li, X.; Wei, T.; Xu, S.; Chen, S.; Cheng, S.H.; Sun, D. Precise drug delivery by using PLGA-based microspheres and optical manipulators. IEEE Tran. Nanobiosci. 2020, 19, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Pho, Q.H.; Wu, X.Y.; Chin, T.Y.; Chen, C.M.; Fang, P.H.; Lin, Y.C.; Hsieh, M.F. PLGA Microspheres loaded with β-cyclodextrin complexes of epigallocatechin-3-gallate for the anti-inflammatory properties in activated microglial cells. Polymers 2018, 10, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Wu, F.; Duan, W.; Mu, X.; Fang, S.; Lu, N.; Zhou, X.; Kong, W. Engineering a “PEG-g-PEI/DNA nanoparticle-in- PLGA microsphere” hybrid controlled release system to enhance immunogenicity of DNA vaccine. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110294. [Google Scholar] [CrossRef]
- Shi, N.Q.; Zhou, J.; Walker, J.; Li, L.; Hong, J.K.Y.; Olsen, K.F.; Tang, J.; Ackermann, R.; Wang, Y.; Qin, B.; et al. Microencapsulation of luteinizing hormone-releasing hormone agonist in poly (lactic-co-glycolic acid) microspheres by spray-drying. J. Control. Release 2020, 321, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; An, N.; Zhang, H.; Zhang, Q.; Zhang, K.; Hu, X.; Wu, Y.; Wu, F.; Xiao, J.; Zhang, H.; et al. Sustained-release of PDGF from PLGA microsphere embedded thermo-sensitive hydrogel promoting wound healing by inhibiting autophagy. J. Drug Deliv. Sci. Technol. 2020, 55, 101405. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, Y.E.; Zhou, X.; Ma, Y.; Guan, S. Long-term sustained release poly(lactic-co-glycolic acid) microspheres of asenapine maleate with improved bioavailability for chronic neuropsychiatric diseases. Drug Deliv. 2020, 27, 1283–1291. [Google Scholar] [CrossRef]
- Jin, L.; Pan, Y.; Pham, A.C.; Boyd, B.J.; Norton, R.S.; Nicolazzo, J.A. Prolonged plasma exposure of the Kv1.3-inhibitory peptide HsTX1[R14A] by subcutaneous administration of a poly(lactic-co-glycolic acid) (PLGA) microsphere formulation. J. Pharm. Sci. 2021, 110, 1182–1188. [Google Scholar] [CrossRef]
- Wan, B.; Andhariya, J.V.; Bao, Q.; Wang, Y.; Zou, Y.; Burgess, D.J. Effect of polymer source on in vitro drug release from PLGA microspheres. Int. J. Pharm. 2021, 607, 120907. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef]
- Virmani, T.; Gupta, J. Pharmaceutical Application of Microspheres: An Approach for the Treatment of Various Diseases. Int. J. Pharm. Sci. Res. 2017, 8, 3257–3259. [Google Scholar] [CrossRef]
- Iqbal, D.N.; Ehtisham-ul-Haque, S.; Ahmad, S.; Arif, K.; Hussain, E.A.; Iqbal, M.; Alshawwa, S.Z.; Abbas, M.; Amjed, N.; Nazir, A. Enhanced antibacterial activity of chitosan, guar gum and polyvinyl alcohol blend matrix loaded with amoxicillin and doxycycline hyclate drugs. Arab. J. Chem. 2021, 14, 103156. [Google Scholar] [CrossRef]
- Yoo, J.; Won, Y.Y. Phenomenology of the initial burst release of drugs from PLGA microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Terukina, T.; Naito, Y.; Tagami, T.; Morikawa, Y.; Henmi, Y.; Prananingrum, W.; Ichikawa, T.; Ozeki, T. The effect of the release behavior of simvastatin from different PLGA particles on bone regeneration in vitro and in vivo: Comparison of simvastatin-loaded PLGA microspheres and nanospheres. J. Drug Deliv. Sci. Technol. 2016, 33, 136–142. [Google Scholar] [CrossRef]
- Li, X.; Chen, K.; Ji, X.; Yuan, X.; Lei, Z.; Ullah, M.W.; Xiao, J.; Yang, G. Microencapsulation of poorly water-soluble finasteride in polyvinyl alcohol/chitosan microspheres as a long-term sustained release system for potential embolization applications. Eng. Sci. 2021, 13, 105–120. [Google Scholar] [CrossRef]
- Misra, R.; Acharya, S.; Dilnawaz, F.; Sahoo, S.K. Sustained antibacterial activity of doxycycline-loaded poly(D,L-lactide-co-glycolide) and poly(ε-caprolactone) nanoparticles. Nanomedicine 2009, 4, 519–530. [Google Scholar] [CrossRef]
- Reinbold, J.; Hierlemann, T.; Hinkel, H.; Müller, I.; Maier, M.E.; Weindl, T.; Schlensak, C.; Wendel, H.P.; Krajewski, S. Development and in vitro characterization of poly(lactide-co-glycolide) microspheres loaded with an antibacterial natural drug for the treatment of long-term bacterial infections. Drug Des. Dev. Ther. 2016, 10, 2823. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing MO2-A12, MO7-A10, and M11-A8, 27th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; p. 282. [Google Scholar]
- Komatsu, D.; Hausen, M.A.; Eri, R.Y.; Leal, V.; Pedrini, F.; Yaksic, C.; Alves, T.F.R.; Chaud, M.V.; Fanelli, C.; Noronha, I.; et al. alternative cutaneous substitutes based on poly(l- co-d,l-lactic acid- co-trimethylene carbonate) with Schinus terebinthifolius Raddi extract designed for skin healing. ACS Omega 2019, 4, 18317–18326. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Kaur, A.; Kaur, T.; Kaur, R. Recent biomedical applications and patents on biodegradable polymer-PLGA. Int. J. Pharm. Sci. 2014, 2, 30–42. [Google Scholar]
- Mateescu, M.; Doncea, S.M.; Chican, I.; Nistor, C.L.; Bujanca, I.C. Synthesis of calcium carbonate micro particles using emulsion membrane process applied for drug release studies. Rev. Chim. 2017, 68, 2320–2324. [Google Scholar] [CrossRef]
- Varde, N.K.; Pack, D.W. Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 2004, 4, 35–51. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Wang, L.; Hao, D.; Ma, G. Fabrication strategy for amphiphilic microcapsules with narrow size distribution by premix membrane emulsification. Colloids Surf. B Biointerfaces 2011, 87, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mundargi, R.C.; Babu, V.R.; Rangaswamy, V.; Patel, P.; Aminabhavi, T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. J. Control. Release 2008, 125, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Teply, B.A.; Jeong, S.Y.; Yim, C.H.; Ho, D.; Sherifi, I.; Jon, S.; Farokhzad, O.C.; Khademhosseini, A.; Langer, R.S. Magnetically responsive polymeric microparticles for oral delivery of protein drugs. Pharm. Res. 2006, 23, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Yang, H.; Su, L.; Yang, Z.; Tang, X. Effect of size on the in vitro/in vivo drug release and degradation of exenatide-loaded PLGA microspheres. J. Drug Deliv. Sci. Technol. 2018, 45, 346–356. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(lactic acid)-based microparticles for drug delivery applications: An overview of recent advances. Pharmaceutics. 2022, 14, 359. [Google Scholar] [CrossRef]
- Gangapurwala, G.; Vollrath, A.; De San Luis, A.; Schubert, U.S. PLA/PLGA-based drug delivery systems produced with supercritical CO2—A green future for particle formulation? Pharmaceutics 2020, 12, 1118. [Google Scholar] [CrossRef]
- Nanaki, S.; Siafaka, P.I.; Zachariadou, D.; Nerantzaki, M.; Giliopoulos, D.J.; Triantafyllidis, K.S.; Kostoglou, M.; Nikolakaki, E.; Bikiaris, D.N. PLGA/SBA-15 mesoporous silica composite microparticles loaded with paclitaxel for local chemotherapy. Eur. J. Pharm. Sci. 2017, 99, 32–44. [Google Scholar] [CrossRef]
- Dawes, G.J.S.; Fratila-Apachitei, L.E.; Mulia, K.; Apachitei, I.; Witkamp, G.J.; Duszczyk, J. Size effect of PLGA spheres on drug loading efficiency and release profiles. J. Mater. Sci. Mater. Med. 2009, 20, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.F.; Yang, B.; Xiong, Q.; Cai, W.W.; Lan, Z.G.; Ying, G.G. Hydrolytic transformation mechanism of tetracycline antibiotics: Reaction kinetics, products identification and determination in WWTPs. Ecotoxicol. Environ. Saf. 2022, 229, 113063. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Tabata, Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011, 7, 2797–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lastra, M.L.; Gómez Ribelles, J.L.; Cortizo, A.M. Design and characterization of microspheres for a 3D mesenchymal stem cell culture. Colloids Surf B Biointerfaces 2020, 196, 111322. [Google Scholar] [CrossRef]
- Ali, M.; Walboomers, X.F.; Jansen, J.A.; Yang, F. Influence of formulation parameters on encapsulation of doxycycline in PLGA microspheres prepared by double emulsion technique for the treatment of periodontitis. J. Drug Deliv. Sci. Technol. 2019, 52, 263–271. [Google Scholar] [CrossRef]



| Sample | Yield |
|---|---|
| PLGA (S) | 75.69 ± 3.71% |
| PLGA (MR) | 77.04 ± 2.87% |
| PLGA + DOX (S) | 71.69 ± 8.21% |
| PLGA + DOX (MR) | 74.01 ± 4.87% |
| Sample | Initial DOX Concentration (mg/L) | Unencapsulated DOX Concentration (mg/L) | Initial DOX Concentration Minus Unencapsulated DOX Concentration (mg/L) | Encapsulation Efficiency (%) | Drug Loading (%) |
|---|---|---|---|---|---|
| (MR) | |||||
| DOX 1 | 38.46 | 8.27 | 30.19 | 78.50 | 41.66 |
| DOX 2 | 48.08 | 8.98 | 39.10 | 81.32 | 33.32 |
| * DOX 3 | 51.28 | 6.69 | 44.59 | 86.96 | 31.25 |
| DOX 4 | 54.49 | 8.71 | 45.78 | 84.01 | 29.41 |
| DOX 5 | 41.67 | 7.71 | 33.96 | 81.50 | 38.47 |
| DOX 6 | 44.87 | 7.77 | 37.10 | 82.69 | 35.70 |
| (S) | |||||
| * DOX 1 | 54.49 | 7.73 | 46.76 | 85.82 | 29.41 |
| DOX 2 | 44.87 | 7.52 | 37.35 | 83.25 | 35.71 |
| DOX 3 | 38.46 | 7.11 | 31.35 | 81.51 | 41.62 |
| DOX 4 | 41.67 | 6.94 | 34.73 | 83.35 | 38.44 |
| DOX 5 | 41.67 | 7.57 | 34.09 | 81.82 | 38.44 |
| DOX 6 | 44.87 | 7.79 | 37.09 | 82.65 | 35.70 |
| Inhibition Zone (mm) | ||||
|---|---|---|---|---|
| Controls | Treated Samples * | |||
| Doxycycline (D) | Gentamicin (G) | PLGA + DOX ** | ||
| Time (h) | Susceptibility *** | |||
| 3 | 30 | 20 | 14 | Intermediate |
| 6 | 13 | Intermediate | ||
| 9 | ||||
| 24 | ||||
| 30 | 10 | Resistant | ||
| 48 | 7 | Resistant | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrini, F.; Nazato, V.S.; Hausen, M.A.; Komatsu, D.; Peña, S.S.; Almeida, A.L.M.; Pirola, F.J.C.; Françoso, M.P.; Duek, E.A.R. In Vitro Cell Behavior and Antibiotic Activity under Sustained Release of Doxycycline-Loaded Poly(lactic-co-glycolic acid) Microspheres. Antibiotics 2022, 11, 945. https://doi.org/10.3390/antibiotics11070945
Pedrini F, Nazato VS, Hausen MA, Komatsu D, Peña SS, Almeida ALM, Pirola FJC, Françoso MP, Duek EAR. In Vitro Cell Behavior and Antibiotic Activity under Sustained Release of Doxycycline-Loaded Poly(lactic-co-glycolic acid) Microspheres. Antibiotics. 2022; 11(7):945. https://doi.org/10.3390/antibiotics11070945
Chicago/Turabian StylePedrini, Flavia, Virgínia S. Nazato, Moema A. Hausen, Daniel Komatsu, Stela S. Peña, Ana Lídia M. Almeida, Fernanda J. C. Pirola, Marina P. Françoso, and Eliana A. R. Duek. 2022. "In Vitro Cell Behavior and Antibiotic Activity under Sustained Release of Doxycycline-Loaded Poly(lactic-co-glycolic acid) Microspheres" Antibiotics 11, no. 7: 945. https://doi.org/10.3390/antibiotics11070945
APA StylePedrini, F., Nazato, V. S., Hausen, M. A., Komatsu, D., Peña, S. S., Almeida, A. L. M., Pirola, F. J. C., Françoso, M. P., & Duek, E. A. R. (2022). In Vitro Cell Behavior and Antibiotic Activity under Sustained Release of Doxycycline-Loaded Poly(lactic-co-glycolic acid) Microspheres. Antibiotics, 11(7), 945. https://doi.org/10.3390/antibiotics11070945









